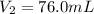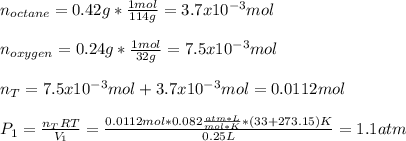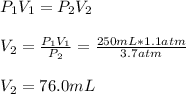Chemistry, 20.10.2020 20:01 GamerGirl15
in a car engine a 250 mL cylinder and piston system is filled with a mixture of 0.42g and 0.24 g of octane gas and oxygen gas, respectively. Both gases are at room temperature of 33 c. If the total gas mixture is compressed to a pressure of 3.7 atm before combustion, calculate the total volume of gas mixture at this point in time.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3
You know the right answer?
in a car engine a 250 mL cylinder and piston system is filled with a mixture of 0.42g and 0.24 g of...
Questions

Mathematics, 01.03.2021 23:00

Biology, 01.03.2021 23:00







English, 01.03.2021 23:00


Social Studies, 01.03.2021 23:00


Mathematics, 01.03.2021 23:00

Physics, 01.03.2021 23:00

Advanced Placement (AP), 01.03.2021 23:00


Health, 01.03.2021 23:00

Physics, 01.03.2021 23:00

Mathematics, 01.03.2021 23:00