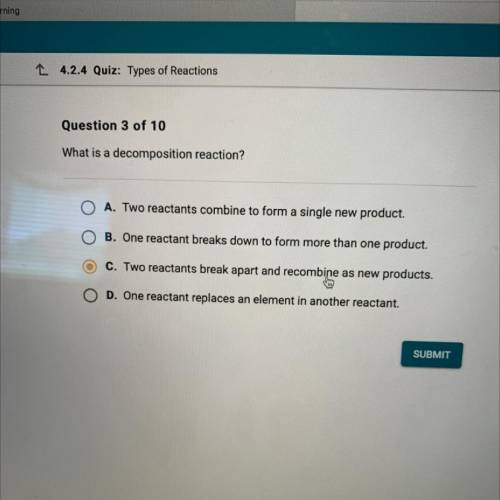
What is a decomposition reaction?
A. Two reactants combine to form a single new product.
B. O...

Chemistry, 21.10.2020 01:01 kparker7543
What is a decomposition reaction?
A. Two reactants combine to form a single new product.
B. One reactant breaks down to form more than one product.
C. Two reactants break apart and recombine as new products.
O D. One reactant replaces an element in another reactant.
SUBMIT
Plzzz help

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Several kinds of bears are found on earth. most bears are brown or black, but one type of bear, the polar bear, is white. what process led to this difference in fur color? explain your answer.
Answers: 1

Chemistry, 22.06.2019 00:00
Which type of bonding involves the complete transfer of a valence electron from a less electrogrative atom to a more electronegative one
Answers: 1


Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1
You know the right answer?
Questions


Computers and Technology, 10.12.2019 23:31











Mathematics, 10.12.2019 23:31



Mathematics, 10.12.2019 23:31



English, 10.12.2019 23:31



