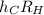Chemistry, 21.10.2020 04:01 logsdonella
With some manipulation, the Rydberg equation can be rewritten in the form
E=constant×(1nf2−1ni2)
which allows you to calculate the energy of the emitted light. Express this constant in terms of the constants h, c, and RH using relationships between wavelength and energy as well as the Rydberg equation from the introduction.
Express the constant in terms of h and c, and RH.
Please help me with this question?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
150.0 grams of asf3 were reacted with 180.0 g of ccl4 to produce ascl3 and ccl2f2. if the actual yield of ccl2f2 was 127 g, what is the percent yield?
Answers: 2

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1
You know the right answer?
With some manipulation, the Rydberg equation can be rewritten in the form
E=constant×(1nf2−1ni2)
Questions




English, 11.03.2020 01:05


Mathematics, 11.03.2020 01:05



Mathematics, 11.03.2020 01:05







Mathematics, 11.03.2020 01:05




French, 11.03.2020 01:06