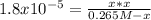Chemistry, 21.10.2020 05:01 maricruzisfye
Consider the reaction: CH3COOH(aq)+H2O(l)⇌H3O+(aq)+CH3COO− (aq) K=1.8×10−5 at 25∘C Part A If a solution initially contains 0.265 molL−1 CH3COOH, what is the equilibrium concentration of H3O+ at 25∘C?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1
You know the right answer?
Consider the reaction: CH3COOH(aq)+H2O(l)⇌H3O+(aq)+CH3COO− (aq) K=1.8×10−5 at 25∘C Part A If a solut...
Questions





Mathematics, 26.05.2021 19:30

Mathematics, 26.05.2021 19:30



Mathematics, 26.05.2021 19:30




Mathematics, 26.05.2021 19:30


Mathematics, 26.05.2021 19:30


Mathematics, 26.05.2021 19:30


Mathematics, 26.05.2021 19:30

![[H_3O]^+=2.18x10^{-3}M](/tpl/images/0827/0667/36cd6.png)
![Ka=\frac{[H_3O^+][CH_3COO^-]}{[CH_3COOH]}](/tpl/images/0827/0667/570cb.png)
 based on the ICE table, in which it equals the concentration of both H3O+ and CH3COO-, we can also write:
based on the ICE table, in which it equals the concentration of both H3O+ and CH3COO-, we can also write: