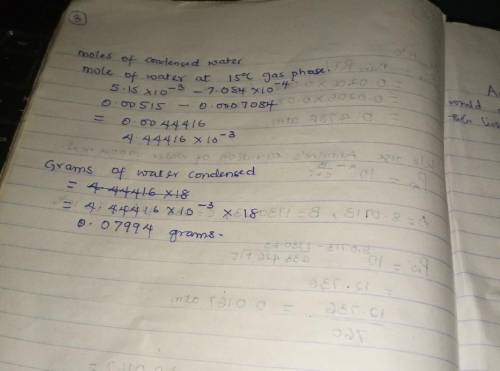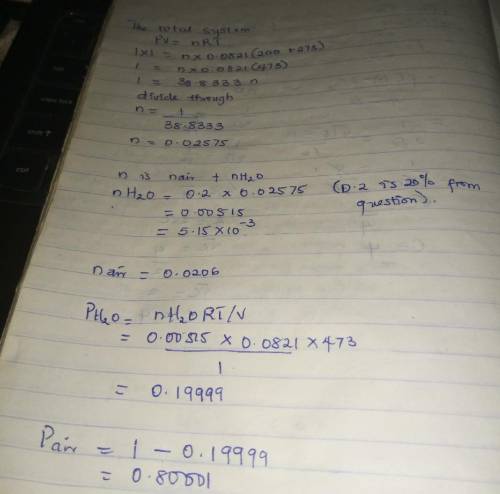Chemistry, 21.10.2020 17:01 smartgirl9989
Air containing 20.0 mol% water vapor at an initial pressure of 1 atm absolute is cooled in a 1- liter sealed vessel from 200°C to 15°C. (a) What is the pressure in the vessel at the end of the process? (Hint: The partial pressure of air in the system can be determined from the expression pair = nairRT/V and P = pair + pH2O. You may neglect the volume of the liquid water condensed, but you must show that condensation occurs.) (b) What is the mole fraction of water in the gas phase at the end of the process? (c) How much water (grams) condenses?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3


Chemistry, 23.06.2019 01:00
If a straight-chain hydrocarbon is a gas at room temperature, how many carbon atoms will it have? a. 6 carbon atoms b. 12 carbon atoms c. 24 carbon atoms d. 3 carbon atoms
Answers: 1

You know the right answer?
Air containing 20.0 mol% water vapor at an initial pressure of 1 atm absolute is cooled in a 1- lite...
Questions

English, 22.03.2021 01:00

Mathematics, 22.03.2021 01:00


Mathematics, 22.03.2021 01:00

Chemistry, 22.03.2021 01:00

Mathematics, 22.03.2021 01:00


Chemistry, 22.03.2021 01:00

Mathematics, 22.03.2021 01:00

Mathematics, 22.03.2021 01:00


Mathematics, 22.03.2021 01:00

History, 22.03.2021 01:00





Biology, 22.03.2021 01:00


Chemistry, 22.03.2021 01:00