
Chemistry, 21.10.2020 18:01 darkdestroyer0888
2.A fisherman in a boat is drinking hot coffee. The large lake below his boat is full of cold water. Which statement is an accurate comparison of the lake water and the coffee?(1 point)
The lake will have more total thermal energy, and the particles in the lake water will be moving faster.
The coffee will have more total thermal energy, but the particles in the lake water will be moving faster.
The lake will have more total thermal energy, but the particles in the coffee will be moving faster.
The coffee will have more total thermal energy, and the particles in the coffee will be moving faster.
3.The gallium in the image is melting in the person’s hand. Which changes will occur on a microscopic level?
(1 point)
The gallium atoms will increase in temperature.
The gallium atoms will gain kinetic energy.
The gallium atoms will gain potential energy, moving freely from each other.
The gallium atoms will be moving faster on average.
4.Which object will have particles with the highest average kinetic energy?(1 point)
an iceberg at 0°C
water at 37°C in a glass
water at 40°C in a swimming pool
a drop of water at 90°C
5. Thermal energy is transferred to a substance. Which change can occur?(1 point)
The particles can gain potential and kinetic energy.
The particles will gain potential energy, but the kinetic energy of the particles will stay the same.
The particles can gain kinetic energy, but the potential energy of the particles will stay the same.
The kinetic and potential energy of the particles will stay the same.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Oxidation-reduction reactions (often called "redox" for short) are reactions that involve the transfer of electrons from one species to another. oxidation states, or oxidation numbers, allow chemists to keep track of these electron transfers. in general, one element will lose electrons (oxidation), with the result that it will increase in oxidation number, and another element will gain electrons (reduction), thereby decreasing in oxidation number. the species that is oxidized is called the reducing agent or reductant. the species that is reduced is called the oxidizing agent or oxidant. to sum up: oxidation = increase in oxidation state = loss of electrons = reducing agent reduction = decrease in oxidation state = gain of electrons = oxidizing agent part a which element is oxidized in this reaction? fe2o3+3co→2fe+3co2 enter the elemental symbol. view available hint(s) is oxidized part b which element is reduced in this reaction? 2hcl+2kmno4+3h2c2o4→6co2+2mno2+2kcl+4h2o enter the elemental symbol. view available hint(s) is reduced
Answers: 1


Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1
You know the right answer?
2.A fisherman in a boat is drinking hot coffee. The large lake below his boat is full of cold water....
Questions


Mathematics, 10.06.2020 22:57

Mathematics, 10.06.2020 22:57

Mathematics, 10.06.2020 22:57


Mathematics, 10.06.2020 22:57

Social Studies, 10.06.2020 22:57

Mathematics, 10.06.2020 22:57

Mathematics, 10.06.2020 22:57



Mathematics, 10.06.2020 22:57

History, 10.06.2020 22:57


Geography, 10.06.2020 22:57


Geography, 10.06.2020 22:57


Mathematics, 10.06.2020 22:57

Mathematics, 10.06.2020 22:57

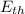 = Kinetic Translational Energy + Kinetic Rotational Energy + Energy due to vibration
= Kinetic Translational Energy + Kinetic Rotational Energy + Energy due to vibration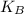 ·T (Monoatomic gas thermal energy)
·T (Monoatomic gas thermal energy)

