
Chemistry, 22.10.2020 19:01 shaking9302
An unknown weak acid with a concentration of 0.079 M has a pH of 1.80. What is the Ka of the weak acid

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

You know the right answer?
An unknown weak acid with a concentration of 0.079 M has a pH of 1.80. What is the Ka of the weak ac...
Questions




Mathematics, 16.12.2020 20:30

Mathematics, 16.12.2020 20:30

Social Studies, 16.12.2020 20:30



Biology, 16.12.2020 20:30



Mathematics, 16.12.2020 20:30








![\frac{[H^+][A^-]}{[HA]}](/tpl/images/0832/0676/f1369.png)
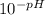 =0.0158
=0.0158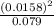 = 3.18x10⁻³
= 3.18x10⁻³ 


