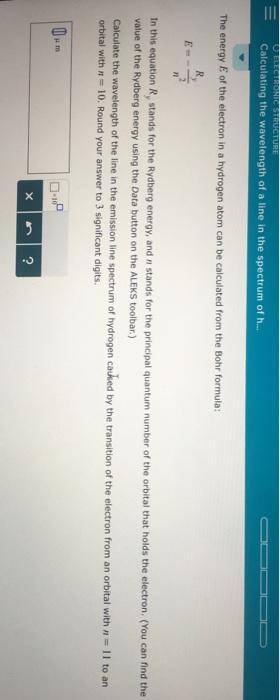
Chemistry, 22.10.2020 20:01 amourrrblackkkk
Calculate the wavelength of the line in the emission line spectrum of hydrogen caused by the transition of the electron from an orbital with to an orbital with . Round your answer to significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1


Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 22.06.2019 22:30
Why is it possible for different microorganisms to extract energy not only from carbohydrates and other biological molecules but from a large variety of substances?
Answers: 1
You know the right answer?
Calculate the wavelength of the line in the emission line spectrum of hydrogen caused by the transit...
Questions



Mathematics, 04.01.2021 01:00


English, 04.01.2021 01:00

English, 04.01.2021 01:00

Mathematics, 04.01.2021 01:00

SAT, 04.01.2021 01:00


Health, 04.01.2021 01:00


Biology, 04.01.2021 01:00

Arts, 04.01.2021 01:00


Mathematics, 04.01.2021 01:00


Arts, 04.01.2021 01:00

English, 04.01.2021 01:00


Mathematics, 04.01.2021 01:00

![[\frac{1}{n_{1} ^2} -\frac{1}{n_{2} ^2} ]](/tpl/images/0832/2451/c2e1d.png)
 = 10
= 10 = 11
= 11