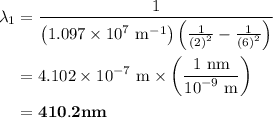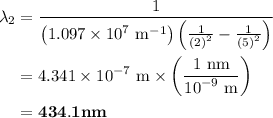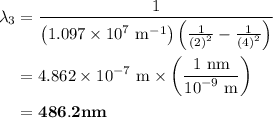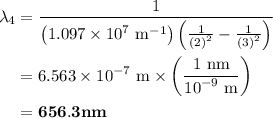Chemistry, 17.09.2019 12:20 lizzyhearts
The spectral lines observed for hydrogen arise from transitions from excited states back to the n=2 principle quantum level. calculate the wavelengths associated with the spectral transitions of the hydrogen atom from the n=6,5,4 and 3 to the n=2 level.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1


Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

Chemistry, 23.06.2019 04:31
Which molecules are more strongly attracted to one another -c3h8o molecules that make up liquid rubbing alcohol or ch4 molecules that make up methane gas
Answers: 3
You know the right answer?
The spectral lines observed for hydrogen arise from transitions from excited states back to the n=2...
Questions

English, 07.07.2019 07:00




Mathematics, 07.07.2019 07:00


Arts, 07.07.2019 07:00



Computers and Technology, 07.07.2019 07:00



Geography, 07.07.2019 07:00

Computers and Technology, 07.07.2019 07:00

Social Studies, 07.07.2019 07:00

History, 07.07.2019 07:00

Mathematics, 07.07.2019 07:00

Mathematics, 07.07.2019 07:00


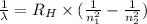
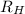 = Rydberg constant =
= Rydberg constant = 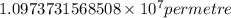
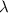 = wavelength
= wavelength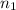 and
and 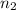 are the level of transitions.
are the level of transitions.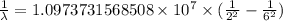
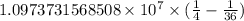
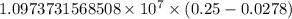
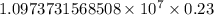
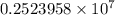
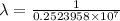


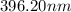
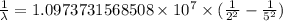
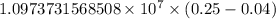
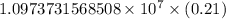
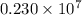
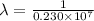


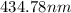
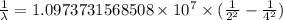
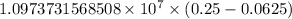
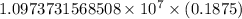
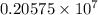
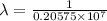



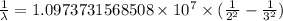
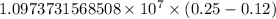
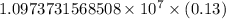
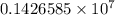
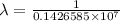


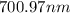
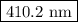 .
.
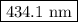 .
.
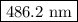 .
.
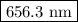 .
.
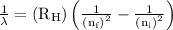 …… (1)
…… (1)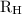 is the Rydberg constant that has the value
is the Rydberg constant that has the value 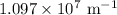 ,
, 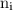 is the initial energy level of transition, and
is the initial energy level of transition, and 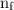 is the final energy level of transition.
is the final energy level of transition.
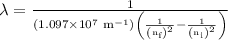 …… (2)
…… (2)