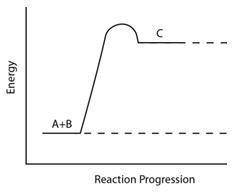
Consider the reaction pathway graph below.
Which statement accurately describes this graph?
A...

Chemistry, 22.10.2020 21:01 evanwall91
Consider the reaction pathway graph below.
Which statement accurately describes this graph?
A) It represents an endothermic reaction because the product has more energy than the reactants.
B) It represents an exothermic reaction because the product has more energy than the reactants.
C) It represents an endothermic reaction because the reactants have more energy than the product.
D) It represents an exothermic reaction because the reactants have more energy than the product.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2

Chemistry, 22.06.2019 23:00
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1
You know the right answer?
Questions







History, 25.02.2020 19:42







Biology, 25.02.2020 19:43



History, 25.02.2020 19:43



Biology, 25.02.2020 19:43



