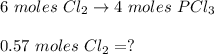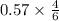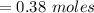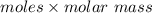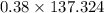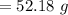Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3
You know the right answer?
A 12.39 g sample of phosphorus reacts with 40.75 g of chlorine to form only phosphorus trichloride (...
Questions




Mathematics, 22.09.2021 01:00

Mathematics, 22.09.2021 01:00


Mathematics, 22.09.2021 01:00

Health, 22.09.2021 01:00


Mathematics, 22.09.2021 01:00


Social Studies, 22.09.2021 01:00


English, 22.09.2021 01:00



History, 22.09.2021 01:00


Mathematics, 22.09.2021 01:00


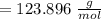
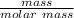
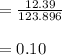
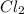 molar mass
molar mass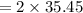
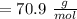
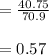
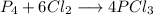
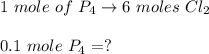
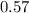 ,
, 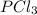 Theoretical performance
Theoretical performance