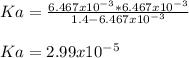Chemistry, 23.10.2020 15:40 osirisarellane3792
The pH of a solution of 4-chlorobutanoic acid is measured to be . Calculate the acid dissociation constant of 4-chlorobutanoic acid. Round your answer to significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 19:30
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2

You know the right answer?
The pH of a solution of 4-chlorobutanoic acid is measured to be . Calculate the acid dissociation co...
Questions

Mathematics, 06.12.2019 20:31


Mathematics, 06.12.2019 20:31


Biology, 06.12.2019 20:31

History, 06.12.2019 20:31



Mathematics, 06.12.2019 20:31


History, 06.12.2019 20:31


History, 06.12.2019 20:31







Social Studies, 06.12.2019 20:31

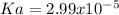
![[H^+]=10^{-pH}=10^{-2.19}=6.467x10^{-3}M](/tpl/images/0835/0688/1103b.png)
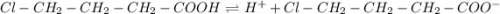
![Ka=\frac{[H^+][Cl-CH_2-CH_2-CH_2-COO^-]}{[Cl-CH_2-CH_2-CH_2-COOH]}](/tpl/images/0835/0688/e99eb.png)
 , the acid dissociation constant is:
, the acid dissociation constant is: