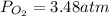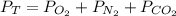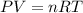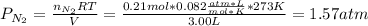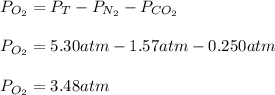Chemistry, 23.10.2020 15:40 kayleahrayne
Suppose that Daniel has a 3.00 L bottle that contains a mixture of O2 , N2 , and CO2 under a total pressure of 5.30 atm. He knows that the mixture contains 0.210 mol N2 and that the partial pressure of CO2 is 0.250 atm. If the temperature is 273 K, what is the partial pressure of O2

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Based on the law of conservation of energy, which statement is false? answer- energy is lost when machines dont work right
Answers: 1

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2

Chemistry, 23.06.2019 08:00
At 35.0°c and 3.00 atm pressure, a gas has a volume of 1.40 l. what pressure does the gas have at 0.00°c and a volume of 0.950 l? which equation should you use? p2= p1v1t2/t1v2what is the pressure of the gas? 3.92 atm these are the answers
Answers: 1
You know the right answer?
Suppose that Daniel has a 3.00 L bottle that contains a mixture of O2 , N2 , and CO2 under a total p...
Questions

Biology, 06.04.2021 19:40





Mathematics, 06.04.2021 19:40

Mathematics, 06.04.2021 19:40

Mathematics, 06.04.2021 19:40

Chemistry, 06.04.2021 19:40


Arts, 06.04.2021 19:40

Spanish, 06.04.2021 19:40







Mathematics, 06.04.2021 19:40