

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1

Chemistry, 23.06.2019 03:00
Determine type of reaction & predict the product c3h12+o2 =
Answers: 1

Chemistry, 23.06.2019 06:10
How much would the freezing point of water decrease if 4 mol of nacl were added to 1 kg of water (kf=1.86 degrees c/(mol/kg) for water and i=2 for nacl a- 7.44 degrees c b- 14.88 c 3.72 d 1.86
Answers: 1

Chemistry, 23.06.2019 07:30
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 1
You know the right answer?
- Using the choices below, which is the complete symbol for the isotope that has the following subat...
Questions

Biology, 18.03.2020 20:25

Mathematics, 18.03.2020 20:25

History, 18.03.2020 20:25

History, 18.03.2020 20:25



Computers and Technology, 18.03.2020 20:26





Mathematics, 18.03.2020 20:26

Chemistry, 18.03.2020 20:26



Physics, 18.03.2020 20:26




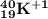 Further explanation
Further explanation


