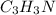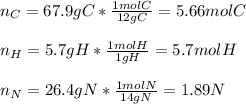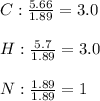Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 19:00
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3

Chemistry, 23.06.2019 03:00
What happens in the particles of a gas when the gas is compressed
Answers: 1
You know the right answer?
Calculate the empirical formula of a compound that contains 67.9% carbon, 5.7 % H and 26.4 % N. When...
Questions


Biology, 13.08.2021 01:10



Mathematics, 13.08.2021 01:10

Physics, 13.08.2021 01:10

History, 13.08.2021 01:10

Mathematics, 13.08.2021 01:10


Social Studies, 13.08.2021 01:10

Mathematics, 13.08.2021 01:10





Mathematics, 13.08.2021 01:10

Mathematics, 13.08.2021 01:10



Mathematics, 13.08.2021 01:10