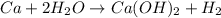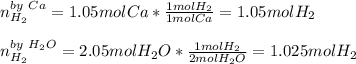Chemistry, 26.10.2020 16:40 SmokyWolves607
In the reaction Ca 2 H2O -> Ca(OH)2 H2, if starting with 1.05 moles of Ca and 2.05 moles of water, which is the limiting reactant

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 10:00
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1

Chemistry, 23.06.2019 03:30
Ahelium balloon contains 16.9 l of helium at stp. how many atoms of helium are in the balloon
Answers: 1
You know the right answer?
In the reaction Ca 2 H2O -> Ca(OH)2 H2, if starting with 1.05 moles of Ca and 2.05 moles of water...
Questions

Spanish, 08.07.2019 14:20



Mathematics, 08.07.2019 14:20


Biology, 08.07.2019 14:20


Biology, 08.07.2019 14:20

Mathematics, 08.07.2019 14:20

Mathematics, 08.07.2019 14:20

Biology, 08.07.2019 14:20

English, 08.07.2019 14:20

Mathematics, 08.07.2019 14:20


Computers and Technology, 08.07.2019 14:20


English, 08.07.2019 14:20

English, 08.07.2019 14:20


Social Studies, 08.07.2019 14:20