Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:00
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 03:00
Select all that apply. a beta particle: is electromagnetic energy is an electron has zero charge is emitted from the nucleus has a +2 charge has a -1 charge
Answers: 1

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2
You know the right answer?
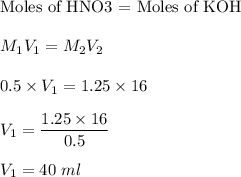
In a titration, what volume of 0.50 M HNO3 is required to completely react with 16.0 mL of 1.250 M K...
Questions

English, 28.01.2020 13:36

English, 28.01.2020 13:36


Mathematics, 28.01.2020 13:37

English, 28.01.2020 13:37

History, 28.01.2020 13:37


Social Studies, 28.01.2020 13:37


Mathematics, 28.01.2020 13:37

Biology, 28.01.2020 13:37



Biology, 28.01.2020 13:37


History, 28.01.2020 13:37

History, 28.01.2020 13:37



Biology, 28.01.2020 13:37