
Chemistry, 26.10.2020 17:20 justinchou814
Calculate the mass defect for 239U239U, which has a mass of 239.05429 amuamu . (The mass of 11H11H is 1.00783 amuamu, and the mass of a neutron is 1.00866 amuamu .)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Aphysical reaction is a process in which one or more reactants change into one or more products with different properties. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

You know the right answer?
Calculate the mass defect for 239U239U, which has a mass of 239.05429 amuamu . (The mass of 11H11H i...
Questions

History, 30.01.2020 14:45



History, 30.01.2020 14:45



Health, 30.01.2020 14:45


Computers and Technology, 30.01.2020 14:45


English, 30.01.2020 14:45


Mathematics, 30.01.2020 14:45

English, 30.01.2020 14:45

Geography, 30.01.2020 14:45


History, 30.01.2020 14:46

Chemistry, 30.01.2020 14:46

Mathematics, 30.01.2020 14:46

Chemistry, 30.01.2020 14:46

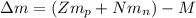
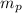 : is the proton mass = 1.00783 amu
: is the proton mass = 1.00783 amu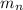 : is the neutron mass = 1.00866 amu
: is the neutron mass = 1.00866 amu ![\Delta m = (Zm_{p} + Nm_{n}) - M = [92*1.00783 amu + (239 - 92)*1.00866 amu] - 239.05429 amu = 1.93909 amu](/tpl/images/0840/4283/ccb79.png)


