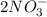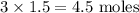When calcium nitrate, Ca(NO3)2, dissolves in water, it dissociates into its ions. We can represent that process by the following equation: Ca(NO3)2(s) → Ca2+(aq) + 2NO3-(aq) When 1.5 mol calcium nitrate dissolves in water, how many moles of ions should be present in solution? Group of answer choices

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1

Chemistry, 23.06.2019 01:30
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1
You know the right answer?
When calcium nitrate, Ca(NO3)2, dissolves in water, it dissociates into its ions. We can represent t...
Questions


Mathematics, 21.10.2019 22:50

Mathematics, 21.10.2019 22:50


Mathematics, 21.10.2019 22:50




Mathematics, 21.10.2019 22:50


Mathematics, 21.10.2019 22:50



Mathematics, 21.10.2019 22:50


Mathematics, 21.10.2019 22:50



English, 21.10.2019 22:50

Physics, 21.10.2019 22:50

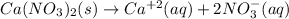
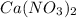 produces 2 moles of
produces 2 moles of 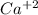 and 1 mole of
and 1 mole of