The image compares the arrangement of electrons in two different neutral atoms.
Atom P:
Atom...

Chemistry, 26.10.2020 19:40 Buttercream16
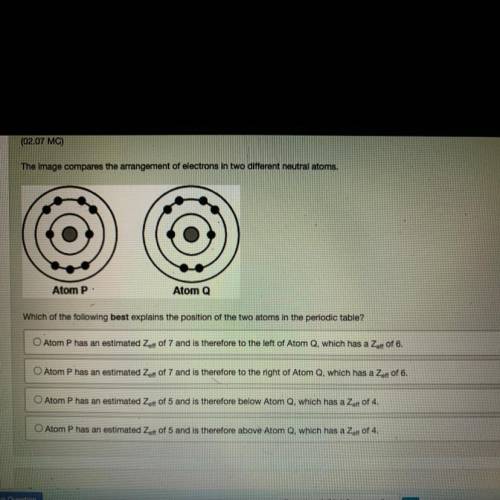
The image compares the arrangement of electrons in two different neutral atoms.
Atom P:
Atom Q
Which of the following best explains the position of the two atoms in the periodic table?
O Atom P has an estimated Zaff of 7 and is therefore to the left of Atom Q, which has a Zert of 6.
O Atom P has an estimated Zeft of 7 and is therefore to the right of Atom Q, which has a Zer of 6.
O Atom P has an estimated Zert of 5 and is therefore below Atom Q, which has a Zoff of 4.
O Atom P has an estimated Zoff of 5 and is therefore above Atom Q, which has a Zet of 4.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1
You know the right answer?
Questions

Social Studies, 17.01.2021 16:50

Mathematics, 17.01.2021 17:00


Arts, 17.01.2021 17:00

Spanish, 17.01.2021 17:00

Mathematics, 17.01.2021 17:00

Mathematics, 17.01.2021 17:00

Mathematics, 17.01.2021 17:00

English, 17.01.2021 17:00


Mathematics, 17.01.2021 17:00

Computers and Technology, 17.01.2021 17:00

Mathematics, 17.01.2021 17:00

History, 17.01.2021 17:00

Computers and Technology, 17.01.2021 17:00


English, 17.01.2021 17:00

Mathematics, 17.01.2021 17:00




