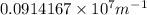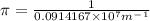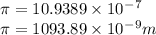Chemistry, 26.10.2020 22:50 oliviaprejean18
Calculate the wavelength of light (in nm) of the spectral line of Hydrogen where an electron falls from the 6th Bohr orbit to the 3rd Bohr orbit.
a) 540 nm
b) 2000 nm
c) 1090 nm
d) 1050 nm

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1

Chemistry, 22.06.2019 21:20
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2
You know the right answer?
Calculate the wavelength of light (in nm) of the spectral line of Hydrogen where an electron falls f...
Questions

Geography, 23.09.2019 14:30



Mathematics, 23.09.2019 14:30


Physics, 23.09.2019 14:30


Social Studies, 23.09.2019 14:30




Mathematics, 23.09.2019 14:30

Biology, 23.09.2019 14:30

Mathematics, 23.09.2019 14:30



Mathematics, 23.09.2019 14:30



Chemistry, 23.09.2019 14:30

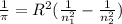
![[n_2n_1]](/tpl/images/0841/8494/b45f5.png)
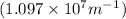
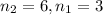
 wavelength of light
wavelength of light 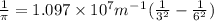
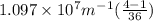
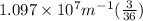
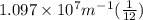
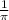 =
=