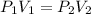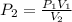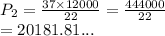Chemistry, 27.10.2020 18:00 cassiuspricerules
A gas sample in a Boyle’s law apparatus is compressed to a volume of 22.0 mL. If its original volume was 37.0 mL at 12,000 Pa, what is the new pressure of the gas sample. Show your calculations.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
The graph above shows how the price of cell phones varies with the demand quantity. the equilibrium price for cell phones is where both supply and demand quantities equal $100, 5,000 5,000, $100
Answers: 2

Chemistry, 21.06.2019 22:30
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 2
You know the right answer?
A gas sample in a Boyle’s law apparatus is compressed to a volume of 22.0 mL. If its original volume...
Questions




Mathematics, 05.05.2020 05:05




History, 05.05.2020 05:05

History, 05.05.2020 05:05


Mathematics, 05.05.2020 05:05








Mathematics, 05.05.2020 05:05