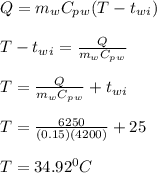Chemistry, 27.10.2020 19:10 Baby010391
A 10.0 g piece of hot metal at 300. °C was dropped into a 150.0 g sample of cooler room temperature water that was initially 25.0 °C. If 6.25 kJ of heat was transferred, what was the final temperature of the water?What was the specific heat of the metal?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1

Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2
You know the right answer?
A 10.0 g piece of hot metal at 300. °C was dropped into a 150.0 g sample of cooler room temperature...
Questions



Advanced Placement (AP), 06.12.2021 04:30



Biology, 06.12.2021 04:30


English, 06.12.2021 04:30

Social Studies, 06.12.2021 04:30

Mathematics, 06.12.2021 04:30



Health, 06.12.2021 04:30


History, 06.12.2021 04:30


English, 06.12.2021 04:30


History, 06.12.2021 04:30

History, 06.12.2021 04:30

 = 300 °C
= 300 °C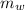 = 150 g = 0.15 kg
= 150 g = 0.15 kg = 25.0 °C
= 25.0 °C