
Chemistry, 15.11.2019 20:31 JessTaylr04
1. nuclear decay by alpha particle emission is more common in atoms of elements that:
a have an atomic number greater than 83
b have a proton to neutron ratio of greater than 3: 1
c have an atomic number less than 83
d have more electrons than protons
2. identify the missing particle in the following equation: 3/2he + 3/2he -> 2 1/1he +
(3/2he refers to 3 above 2 next to he as an isotope symbol)
a 2/1he
b 1/1he
c 5/3li
d 4/2he
3. one of the major drawbacks to using nuclear fission as a reliable energy source is:
a there is not enough energy produced by this method to make it profitable.
b the process cannot be well-controlled.
c the intense amount of heat require to initiate the reaction.
d the products are more radioactive than the reactants.
4. which of the following concerns apply to nuclear fission reactions?
a concern about the disposal of nuclear waste
b concern about the safe operation of nuclear plants
c concern about nuclear weapons proliferation
d all of these are concerns
5. the energy output of the sun and other stars is a result of fission reactions among hydrogen nuclei.
true
false
6. why is the total mass of a helium nucleus not equal to the mass of its individual parts? (points : 3)
a the "missing" mass is due to inaccurate measurement on a very small scale.
b the "missing" mass has been converted to energy.
c the "missing" matter has been converted to mass.
d the "missing" mass has been converted to matter.
7. in a nuclear reaction, the mass-energy of the reactants is generally: (points : 3)
a less than the mass-energy of the products
b equal to the mass of the products
c equal to the energy of the products
d equal to the mass-energy of the products
8. a mass of 10.0 kilograms is completely converted into energy. what is this amount of energy in joules? (1 j = 1 kg m2/s2) (points : 3)
a 3.33 x 10^8 j
b 1.11 x 10^-8 j
c 3.00 x 10^16 j
d 9.00 x 10^17
9. a mass of 4.00 kilograms is completely converted into energy. what is this amount of energy in joules? (1 j = 1 kg m2/s2) (points : 3)
a 1.20 x 10^9 j
b 1.33 x 10^-8 j
c 3.60 x 10^17 j
d 4.4 x 10^-16 j
10. according to einstein's formula for the conversion of mass and energy, to find the energy, multiply the mass by the speed of light. (points : 2)
true
false

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
219 grams of iron (iii) oxide reacts with excess carbon according to the reaction equation shown below. fe2o3 + c → fe + co2 after a scientist performs the chemical reaction they find the actual yield of iron to be 57.4 grams. calculate the percent yield of this chemical reaction.
Answers: 1


Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2

Chemistry, 22.06.2019 21:50
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1
You know the right answer?
1. nuclear decay by alpha particle emission is more common in atoms of elements that:
a have...
a have...
Questions

English, 22.12.2019 23:31

Mathematics, 22.12.2019 23:31

Physics, 22.12.2019 23:31

Chemistry, 22.12.2019 23:31

Mathematics, 22.12.2019 23:31




Chemistry, 22.12.2019 23:31


English, 22.12.2019 23:31


Mathematics, 22.12.2019 23:31


Mathematics, 22.12.2019 23:31



Chemistry, 22.12.2019 23:31

Health, 22.12.2019 23:31

Mathematics, 22.12.2019 23:31

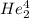
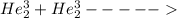
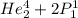
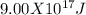
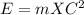 formula can be used.
formula can be used.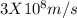
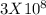 =
= 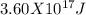 .
.

