Chemistry, 27.10.2020 21:30 mbonham481
A sample of Ar gas has a pressure of 1.20 atm at 125°C and a volume of 1.00 L. What would the temperature be (°C) with a pressure of 1.80 atm and constant volume?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Pls! plant cells and animal cells were observed under a microscope. the characteristics of two cells are listed below. cell p: does not capture sunlight cell q: has cytoplasm but no chloroplast which statement about the two cells is correct? cell q also has a cell wall. cell q also has large vacuole. cell p also has a large vacuole. cell p also has a cell membrane.
Answers: 1

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 23.06.2019 01:00
What is the chemical name of the compound ti2o3? use the list of polyatomic ions and the periodic table to you answer.
Answers: 1

You know the right answer?
A sample of Ar gas has a pressure of 1.20 atm at 125°C and a volume of 1.00 L. What would the temper...
Questions

Biology, 03.08.2019 18:00



History, 03.08.2019 18:00

Physics, 03.08.2019 18:00




History, 03.08.2019 18:00



Mathematics, 03.08.2019 18:00



Business, 03.08.2019 18:00


Mathematics, 03.08.2019 18:00


History, 03.08.2019 18:00

Mathematics, 03.08.2019 18:00

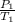 =
= 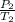
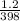 =
=