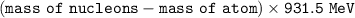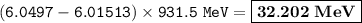Answers: 1


Another question on Chemistry

Chemistry, 20.06.2019 18:04
Why is it illegal to manufacture fireworks without a license
Answers: 1

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1
You know the right answer?
PLEASEEE SOS HELPPP
An atom of lithium-6 has a mass of 6.01513 amu. The sum of the masses of this i...
Questions



Social Studies, 07.10.2020 14:01



Mathematics, 07.10.2020 14:01



Mathematics, 07.10.2020 14:01

Business, 07.10.2020 14:01


Mathematics, 07.10.2020 14:01

Mathematics, 07.10.2020 14:01

Mathematics, 07.10.2020 14:01


History, 07.10.2020 14:01

Business, 07.10.2020 14:01