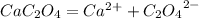Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2


Chemistry, 23.06.2019 00:00
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3
You know the right answer?
Calcium oxalate (cac2o4) has a ksp value of 2.3 × 10–9 at 25°c. calculate the molar solubility of ca...
Questions


Mathematics, 29.01.2021 03:40



Mathematics, 29.01.2021 03:40

Physics, 29.01.2021 03:40

English, 29.01.2021 03:40




History, 29.01.2021 03:40

History, 29.01.2021 03:40

Biology, 29.01.2021 03:40


Computers and Technology, 29.01.2021 03:40

Mathematics, 29.01.2021 03:40

History, 29.01.2021 03:40

Mathematics, 29.01.2021 03:40


Mathematics, 29.01.2021 03:40