Chemistry, 17.09.2019 04:30 kyliegriffis
Considering the following precipitation reaction: pb(no3)2(aq) + 2ki(aq) → pbi2(s) + 2kno3(aq),
which ion(s) would not be spectator ions?
a) pb2+ , no3-
b) k+ , i
c) no3- , pb2+
d) pb2+, i –
e) all the above ions are in the net ionic equation

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:10
When will le chatelier's principle come into effect? at the beginning of a reaction, when there are only reactants when a reaction has reached chemical equilibrium when a catalyst is added to a reaction mixture when a reaction is occurring but not yet at equilibrium
Answers: 3


Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1
You know the right answer?
Considering the following precipitation reaction: pb(no3)2(aq) + 2ki(aq) → pbi2(s) + 2kno3(aq),
Questions

English, 21.04.2021 03:20



Mathematics, 21.04.2021 03:20







History, 21.04.2021 03:20



English, 21.04.2021 03:20



Mathematics, 21.04.2021 03:20

Mathematics, 21.04.2021 03:20

Mathematics, 21.04.2021 03:20

Mathematics, 21.04.2021 03:30

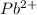 and
and 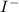
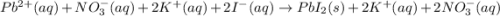
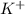 and
and 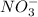 and are spectator ions.
and are spectator ions.