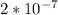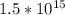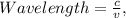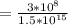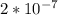Chemistry, 28.10.2020 01:00 michelerin9486
Calculate the wavelength and energy of light that has a frequency of 1.5 x 10^15 Hz.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
150.0 grams of asf3 were reacted with 180.0 g of ccl4 to produce ascl3 and ccl2f2. if the actual yield of ccl2f2 was 127 g, what is the percent yield?
Answers: 2

Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 23.06.2019 12:30
What would happen to a weak base dissociation equilibrium if more products we added
Answers: 1
You know the right answer?
Calculate the wavelength and energy of light that has a frequency of 1.5 x 10^15 Hz....
Questions

Biology, 06.07.2021 16:50



Mathematics, 06.07.2021 16:50


Mathematics, 06.07.2021 16:50

Mathematics, 06.07.2021 17:00



Mathematics, 06.07.2021 17:00


World Languages, 06.07.2021 17:00



Chemistry, 06.07.2021 17:00


Mathematics, 06.07.2021 17:00

Social Studies, 06.07.2021 17:00

Chemistry, 06.07.2021 17:00

Mathematics, 06.07.2021 17:00