
Chemistry, 28.10.2020 06:00 clairebear65
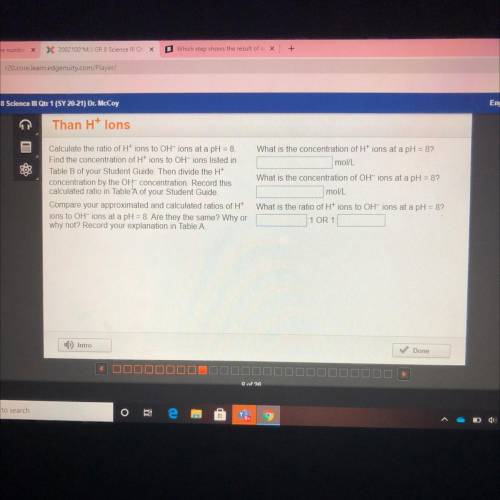
What is the concentration of H+ ions at a pH = 8?
mol/L
Calculate the ratio of Htions to OH-ions at a pH = 8
Find the concentration of H+ ions to OH-ions listed in
Table B of your Student Guide. Then divide the H+
concentration by the OH concentration. Record this
calculated ratio in Table A of your Student Guide.
Compare your approximated and calculated ratios of H+
ions to OH-ions at a pH = 8. Are they the same? Why or
why not? Record your explanation in Table A.
What is the concentration of OH-ions at a pH = 8?
mol/L
What is the ratio of H+ ions to OH-ions at a pH = 8?
1 OR 1.

Answers: 3


Another question on Chemistry


Chemistry, 23.06.2019 02:00
Scientists are often interested in knowing the molar heat of combustion – the heat released during the combustion of one mole of a substance. use the periodic table to find molar masses. how many moles of ethanol are present in the sample?
Answers: 2

Chemistry, 23.06.2019 04:00
Which method would be best to separate a mixture of sand and gravel
Answers: 1

Chemistry, 23.06.2019 06:40
8. how much enthalpy/heat is transferred when 0.5113gof ammonia (nh3) reacts with excess oxygen according| to the following equation: 4nh3 +502 - 4n0+ 6h20ah = -905.4j
Answers: 1
You know the right answer?
What is the concentration of H+ ions at a pH = 8?
mol/L
Calculate the ratio of Htions to OH-i...
Calculate the ratio of Htions to OH-i...
Questions

Mathematics, 11.11.2020 06:00

Mathematics, 11.11.2020 06:00



Geography, 11.11.2020 06:00

Spanish, 11.11.2020 06:00


History, 11.11.2020 06:00






Physics, 11.11.2020 06:00

Health, 11.11.2020 06:00

Mathematics, 11.11.2020 06:00

Mathematics, 11.11.2020 06:00

Mathematics, 11.11.2020 06:00





