

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Straightforward questions answered in the powerpoint slidesreaction: heating the starting materials under refluxwhat does it mean to heat under reflux? why do we choose water as the reflux solvent? what are boiling chips used for? why do we put a condenser on top of the reaction? why do we add heat and let the reaction stir for 30 minutes? why do we add sulfuric acid to the reaction after it cools as opposed to when it’s still hot? separation: filtration of precipitatewhy don’t we do an aqueous and organic extraction in the separatory funnel? why do you rinse the salicylic acid on the filter with ice cold water? purification: recrystallization of salicylic acid (no hot filtration needed)what is the difference in the amount of room temperature water vs. boiling water needed to dissolve the salicylic acid (assume a 1.2 gram yield of salicylic acid)? remember, in the lab if you need x ml of boiling water to dissolve a solid, then you should add a little more (definitely no more than 1.5 times the theoretical amount) to ensure it doesn’t recrystallize prematurely.analysis: melting point of salicylic acidwhat can you conclude if the melting point of the salicylic acid you just synthesized is 152-155oc and the 1: 1 mix of your product and “synthetic” salicylic acid is 151-154oc?
Answers: 1

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
You know the right answer?
Chromium (III) oxide reacts with hydrogen sulfide gas to form chromium (III) sulfide and water. a)wr...
Questions


History, 03.04.2020 03:02



Social Studies, 03.04.2020 03:02



History, 03.04.2020 03:02

Health, 03.04.2020 03:02


Mathematics, 03.04.2020 03:02

Mathematics, 03.04.2020 03:03


History, 03.04.2020 03:03


French, 03.04.2020 03:03

Mathematics, 03.04.2020 03:03


Mathematics, 03.04.2020 03:03

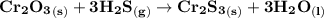
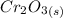 is being required.
is being required.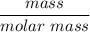
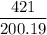
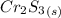 requires 1 mole of
requires 1 mole of 

