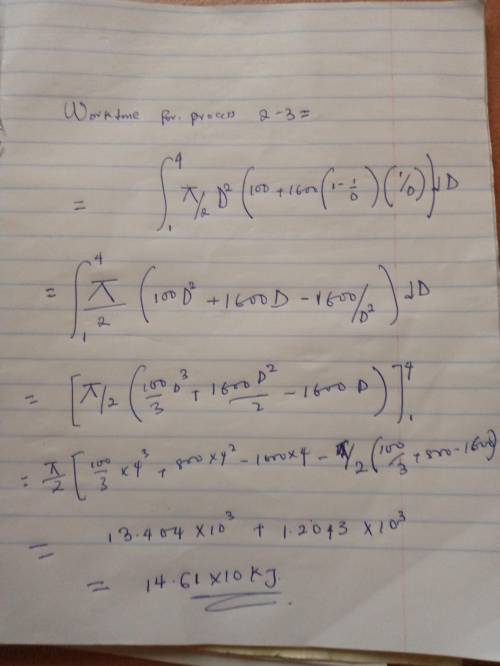Chemistry, 28.10.2020 16:40 adambbogard1589
An initially deflated and flat balloon is connected by a valve to a storage tank containing helium gas at 1 MPa at ambient temperature of 20 degrees C. The valve is open and the balloon is inflated at constant pressure of 100 kPa (atmospheric pressure) until it becomes spherical at D1 = 1m. If the balloon is larger than this, the balloon material is stretched giving a pressure inside as:
P = Po + C(1-(D1/D))(D1/D)
The balloon is slowly inflated to a final diameter of 4m, at which point the pressure inside is 400 kPa. The temperature remains constant at 20 degrees C. Determine the work done during the overall process.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Determine the number o moles of ions/atoms/particle in the following: 2.50 miles of k2s (let me know how to do)
Answers: 1

Chemistry, 23.06.2019 01:30
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1

Chemistry, 23.06.2019 02:00
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1

Chemistry, 23.06.2019 07:30
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes no correct anwser get brainliest
Answers: 1
You know the right answer?
An initially deflated and flat balloon is connected by a valve to a storage tank containing helium g...
Questions



Mathematics, 30.12.2019 20:31


English, 30.12.2019 20:31

Mathematics, 30.12.2019 20:31




Mathematics, 30.12.2019 20:31

Physics, 30.12.2019 20:31





Advanced Placement (AP), 30.12.2019 20:31

Mathematics, 30.12.2019 20:31

Chemistry, 30.12.2019 20:31

Mathematics, 30.12.2019 20:31

Mathematics, 30.12.2019 20:31