
Chemistry, 28.10.2020 17:00 ummmmmmmmmmmm
An iron ore sample contains plus other impurities. A 896-g sample of impure iron ore is heated with excess carbon, producing 182 g of pure iron by the following reaction: What is the mass percent of FeO2 in the impure iron ore sample

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:20
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1

Chemistry, 22.06.2019 02:30
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3
You know the right answer?
An iron ore sample contains plus other impurities. A 896-g sample of impure iron ore is heated with...
Questions




Biology, 21.09.2019 11:50

Physics, 21.09.2019 11:50


Mathematics, 21.09.2019 11:50


Physics, 21.09.2019 11:50

World Languages, 21.09.2019 11:50

History, 21.09.2019 11:50

Mathematics, 21.09.2019 11:50




Social Studies, 21.09.2019 11:50


Computers and Technology, 21.09.2019 11:50


Advanced Placement (AP), 21.09.2019 11:50

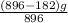 × 100
× 100 

