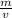Chemistry, 29.10.2020 16:10 jdkrisdaimcc11
A solid piece of lead has a mass of 36.24 g and a volume of 3.22 cm3. From these data, calculate the density of lead in SI units (kilograms per cubic meter).

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:30
If a 60-g object has a volume of 30 cm3, what is its density? 2 g/cm3 0.5 cm3/g 1800 g * cm3 none of the above
Answers: 3

Chemistry, 21.06.2019 23:50
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3
You know the right answer?
A solid piece of lead has a mass of 36.24 g and a volume of 3.22 cm3. From these data, calculate the...
Questions



Mathematics, 20.11.2020 21:20


Mathematics, 20.11.2020 21:20

History, 20.11.2020 21:20


Mathematics, 20.11.2020 21:20

Mathematics, 20.11.2020 21:20









Mathematics, 20.11.2020 21:20


English, 20.11.2020 21:20