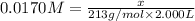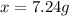Chemistry, 29.10.2020 16:40 adearing2426
a) What mass of aluminium nitrate (Al(NO3)3) would be required to prepare 2.000 L of a 0.0170 M aqueous solution of this salt

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 01:30
Pls! plant cells and animal cells were observed under a microscope. the characteristics of two cells are listed below. cell p: does not capture sunlight cell q: has cytoplasm but no chloroplast which statement about the two cells is correct? cell q also has a cell wall. cell q also has large vacuole. cell p also has a large vacuole. cell p also has a cell membrane.
Answers: 1

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3
You know the right answer?
a) What mass of aluminium nitrate (Al(NO3)3) would be required to prepare 2.000 L of a 0.0170 M aque...
Questions

Biology, 04.11.2021 09:40


Chemistry, 04.11.2021 09:40

History, 04.11.2021 09:40

English, 04.11.2021 09:40

Engineering, 04.11.2021 09:40






Biology, 04.11.2021 09:40

Mathematics, 04.11.2021 09:40

Mathematics, 04.11.2021 09:40

Mathematics, 04.11.2021 09:40


Mathematics, 04.11.2021 09:40

Mathematics, 04.11.2021 09:40


History, 04.11.2021 09:40

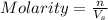
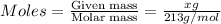
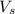 = volume of solution in Liter = 2.000 L
= volume of solution in Liter = 2.000 L