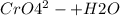Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1


Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 1

Chemistry, 23.06.2019 01:30
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
) Chromate/Dichromate Equilibrium. Yellow chromate ion reacts with hydrogen ion to form (by condensa...
Questions

Mathematics, 28.02.2020 20:27


Mathematics, 28.02.2020 20:27



Business, 28.02.2020 20:27







English, 28.02.2020 20:27

English, 28.02.2020 20:27


History, 28.02.2020 20:27




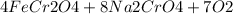 →
→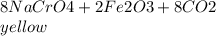
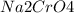 is called Chromite ore which is of yellow color.
is called Chromite ore which is of yellow color.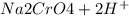 →
→ 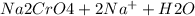
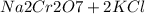 →
→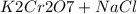
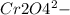
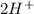 →
→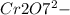
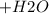
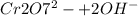 →
→