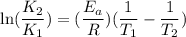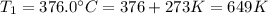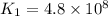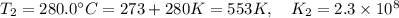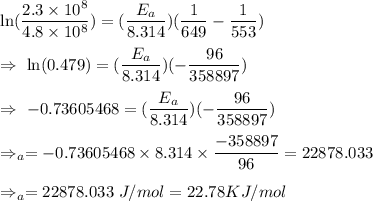The rate constant for a certain reaction is measured at two different temperatures:
Temperature K
376.0°C 4.8 x 10^8
280°C 2.3 x 10^8
Assuming the rate constant obeys the Arrhenius equation, calculate the activation energy for this reaction. Round your answer to significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1


Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1

Chemistry, 23.06.2019 00:00
This statement about matter and its behavior is best classified as a
Answers: 1
You know the right answer?
The rate constant for a certain reaction is measured at two different temperatures:
Temperature K
Questions

History, 05.07.2019 19:50



Computers and Technology, 05.07.2019 19:50

Computers and Technology, 05.07.2019 19:50

Computers and Technology, 05.07.2019 19:50

Computers and Technology, 05.07.2019 19:50

Physics, 05.07.2019 19:50

Physics, 05.07.2019 19:50

Mathematics, 05.07.2019 19:50


Mathematics, 05.07.2019 19:50

Mathematics, 05.07.2019 19:50

Social Studies, 05.07.2019 19:50




Biology, 05.07.2019 19:50

Business, 05.07.2019 19:50

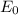 follows by formula:
follows by formula: