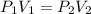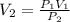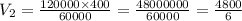Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2
You know the right answer?
The pressure of a 400 mL sample of KNO₂(g) at constant temperature is increased from 120 kPa to 60 k...
Questions


SAT, 28.06.2021 14:00



Mathematics, 28.06.2021 14:00

Mathematics, 28.06.2021 14:00

Mathematics, 28.06.2021 14:00

Advanced Placement (AP), 28.06.2021 14:00






English, 28.06.2021 14:00

Physics, 28.06.2021 14:00

Mathematics, 28.06.2021 14:00

English, 28.06.2021 14:00

English, 28.06.2021 14:00