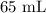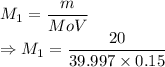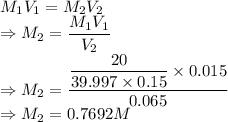Chemistry, 30.10.2020 16:50 kaelah6846
A student prepared a stock solution by dissolving 20.0 g of NaOH in enough water to make 150. mL of solution. She then took 15.0 mL of the stock solution and diluted it with enough water to make 65.0 mL of a final solution. What is the concentration of NaOH for the final solution?
A) O. 411 M
B) 0.534 M
C) 1.87 M
D) 2.43 M

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Un cierto gas tiene un volumen de 800ml a 80°c y 600ml a 80°c y 600mmhg de presión. ¿cual será el volumen del gas a condiciones normales? sí el gas es oxígeno, ¿cuál será su peso? y ¿cuántas moléculas están presentes en el sistema?
Answers: 2


Chemistry, 23.06.2019 12:30
D5w is shorthand for a 5% glucose rehydration fluid used in ivs. the doctor orders 1200 ml d5w@ 30 gtts/min. you have an iv tube that delivers 18 gtts/cc. how many hours will it take for the 1200 cc bottle to infuse?
Answers: 2

Chemistry, 24.06.2019 00:00
Adam wants to work in the field of forensic science and follow the rules for handling evidence. which organization can he join to fulfill this aspiration? international academy of forensic science national academy of sciences national academy of forensic science national science academy
Answers: 2
You know the right answer?
A student prepared a stock solution by dissolving 20.0 g of NaOH in enough water to make 150. mL of...
Questions

Mathematics, 03.02.2021 05:30

English, 03.02.2021 05:30

Physics, 03.02.2021 05:30

Mathematics, 03.02.2021 05:30


Mathematics, 03.02.2021 05:30

Mathematics, 03.02.2021 05:30

Mathematics, 03.02.2021 05:30



English, 03.02.2021 05:30

Mathematics, 03.02.2021 05:30

Mathematics, 03.02.2021 05:30

Mathematics, 03.02.2021 05:30

History, 03.02.2021 05:30

Chemistry, 03.02.2021 05:30

Mathematics, 03.02.2021 05:30



Arts, 03.02.2021 05:30

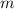 = Mass of sample =
= Mass of sample = 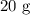
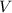 = Volume of solution =
= Volume of solution = 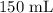
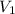 = Initial volume taken out of the stock solution =
= Initial volume taken out of the stock solution = 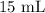
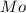 = Molar mass of NaOH =
= Molar mass of NaOH = 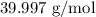
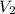 = Final volume of solution =
= Final volume of solution =