b. Is a large mug. It is 120°F

Chemistry, 30.10.2020 23:30 dragongacha777
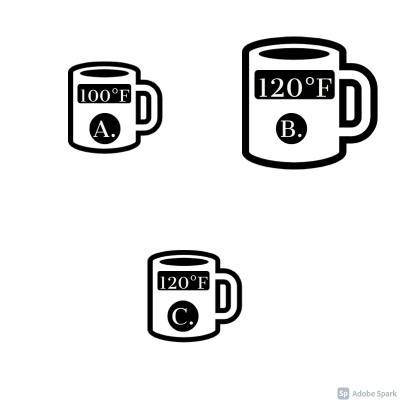
You have three mugs of tea:
a. Is a small mug. It is 100°F
b. Is a large mug. It is 120°F
c. Is a small mug. It is 120°F
Put these mugs in order. Start with the mug with the SMALLEST amount of thermal energy first and end with the mug with the LARGEST amount of thermal energy.
__2__ C. is a small mug. It is 120°F
__3__ A. is a small mug. It is 100°F
__1__ B. is a large mug. It is 120°F

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2
You know the right answer?
You have three mugs of tea:
a. Is a small mug. It is 100°F
b. Is a large mug. It is 120°F
b. Is a large mug. It is 120°F
Questions

Social Studies, 10.11.2020 01:10

Mathematics, 10.11.2020 01:10

Law, 10.11.2020 01:10

Computers and Technology, 10.11.2020 01:10

Arts, 10.11.2020 01:10




Physics, 10.11.2020 01:10


Mathematics, 10.11.2020 01:10




Geography, 10.11.2020 01:10




Mathematics, 10.11.2020 01:10

Mathematics, 10.11.2020 01:10



