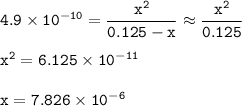Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 03:00
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 22:30
What must be in balance for temperatures to remain constant?
Answers: 1
You know the right answer?
15) What is the hydronium ion concentration [H3O + ] of a 0.125 M hydrocyanic acid solution with Ka...
Questions

Biology, 23.07.2019 08:30

Mathematics, 23.07.2019 08:30





History, 23.07.2019 08:30

Biology, 23.07.2019 08:30



Mathematics, 23.07.2019 08:30


Mathematics, 23.07.2019 08:30

English, 23.07.2019 08:30






![\large {\boxed {\bold {Ka \: = \: \frac {[H ^ +] [A ^ -]} {[HA]}}}}](/tpl/images/0858/5891/560b5.png)
![\tt Ka=\dfrac{[H_3O^+][CN^-]}{[HCN^-]}](/tpl/images/0858/5891/535c0.png)