
Chemistry, 01.11.2020 06:40 nadiarose6366
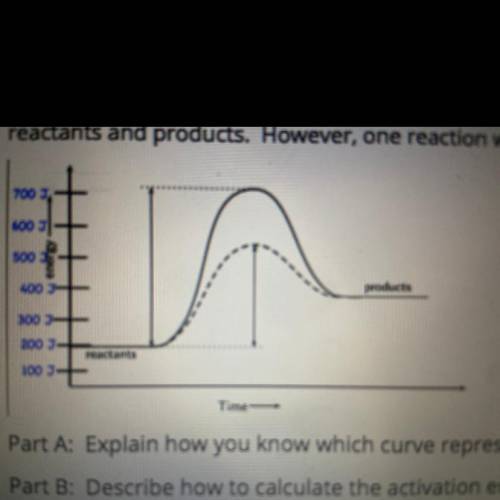
The graph below shows two reactions run in a laboratory. The vertical axis indicates the amount of energy in units of joules. Each reaction involved the same quantities of
reactants and products. However, one reaction was run with a catalyst, and one was run without a catalyst:
Part A Explain how you know which curve represents the reaction with the catalyst and which represents the reaction without the catalyst.
Part B: Describe how to calculate the activation energy with the catalyst, and state the activation energy, in joules.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Balance this equation co2(g) + h2o (g) show that the balanced equation obeys the law if conversation of mass
Answers: 1

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 23.06.2019 00:30
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1
You know the right answer?
The graph below shows two reactions run in a laboratory. The vertical axis indicates the amount of e...
Questions

Mathematics, 15.10.2019 00:10

Mathematics, 15.10.2019 00:10

Mathematics, 15.10.2019 00:10

Chemistry, 15.10.2019 00:10

History, 15.10.2019 00:10





Social Studies, 15.10.2019 00:10



Physics, 15.10.2019 00:10

History, 15.10.2019 00:10

Chemistry, 15.10.2019 00:10







