
Chemistry, 01.11.2020 07:30 briabrodnax19
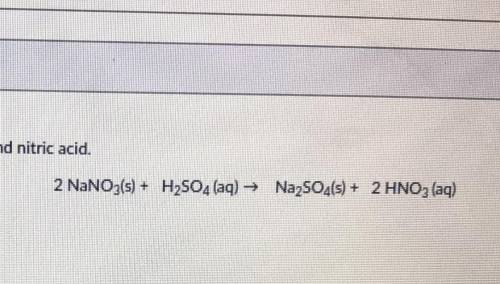
PLEASE HELP! 30 POINTS!Solid sodium nitrate reacts with sulfuric acid to produce sodium sulfate and nitric acid. You have 36 grams of sodium nitrate and 21 grams of sulfuric acid. What is the maximum amount of precipitate that can be formed?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Chemistry, 22.06.2019 21:50
Answer the questions about this reaction: nai(aq) + cl2(g) → nacl(aq) + i2(g) write the oxidation and reduction half-reactions: oxidation half-reaction: reduction half-reaction: based on the table of relative strengths of oxidizing and reducing agents (b-18), would these reactants form these products? write the balanced equation: answer options: a. 0/na -> +1/na+1e- b. nai(aq) + cl2(g) → nacl(aq) + i2(g) c. +1/na+1e- -> 0 /na d. -1/2i -> 0/i2+2e- e. no f. 4nai(aq) + cl2(g) → 4nacl(aq) + i2(g) g. 2nai(aq) + cl2(g) → 2nacl(aq) + i2(g) h. 4nai(aq) + 2cl2(g) → 4nacl(aq) + 2i2(g) i. nai(aq) + cl2(g) → nacl(aq) + i2(g) j. 0/cl2+2e -> -1/2cl- k. yes
Answers: 1

You know the right answer?
PLEASE HELP! 30 POINTS!Solid sodium nitrate reacts with sulfuric acid to produce sodium sulfate and...
Questions


Mathematics, 24.03.2021 17:20

Mathematics, 24.03.2021 17:20




Mathematics, 24.03.2021 17:20

SAT, 24.03.2021 17:20



Biology, 24.03.2021 17:20

Mathematics, 24.03.2021 17:20




Mathematics, 24.03.2021 17:20






