
Chemistry, 02.11.2020 05:20 loveuncondition
Solid magnesium, Mg, reacts with oxygen gas, 02 to form the solid magnesium oxide, MgO. Which is a balanced chemical equation for this reaction?
A. Mg + MgO
B. Mg + O2 + MgO,
C. Mg + 20 2MgO
D. 2Mg +0,- 2MgO
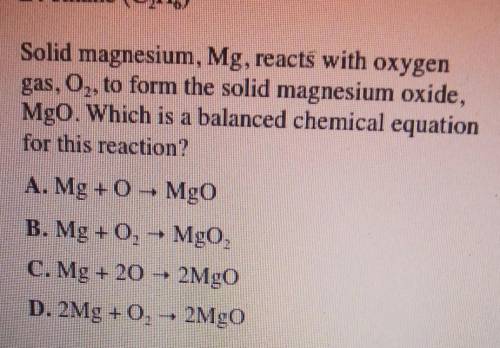

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 23.06.2019 02:40
Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following information: c(graphite) + o2 latex: \longrightarrow ⟶ co2(g) latex: \delta δ h° = –393.5 kj/mol h2(g) + o2 latex: \longrightarrow ⟶ h2o(l) latex: \delta δ h° = –285.8 kj/mol ch3oh(l) + o2(g) latex: \longrightarrow ⟶ co2(g) + 2h2o(l) latex: \delta δ h° = –726.4 kj/mol
Answers: 3

Chemistry, 23.06.2019 07:30
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 2
You know the right answer?
Solid magnesium, Mg, reacts with oxygen gas, 02 to form the solid magnesium oxide, MgO. Which is a b...
Questions




History, 24.08.2020 08:01



Mathematics, 24.08.2020 08:01


History, 24.08.2020 08:01


Mathematics, 24.08.2020 08:01


History, 24.08.2020 08:01







Mathematics, 24.08.2020 08:01



