
Chemistry, 02.11.2020 05:50 ghari112345
PLEASE HELP! According to the equation, how many liters of oxygen gas at STP are produced when 2.00 moles of potassium chlorate decomposes?
a. 134 L
b. 67.2 L
c. 22.4 L
d. 11.2 L
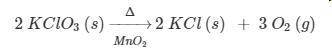

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1
You know the right answer?
PLEASE HELP! According to the equation, how many liters of oxygen gas at STP are produced when 2.00...
Questions

History, 13.07.2019 22:30



Health, 13.07.2019 22:30

Advanced Placement (AP), 13.07.2019 22:30

Health, 13.07.2019 22:30

English, 13.07.2019 22:30

Physics, 13.07.2019 22:30

English, 13.07.2019 22:30






Mathematics, 13.07.2019 22:30


History, 13.07.2019 22:30





