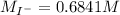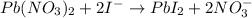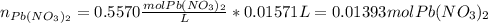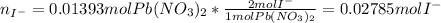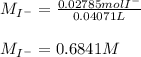Chemistry, 02.11.2020 16:30 KillerMDFK7992
The iodide ion concentration in a solution may be determined by the precipitation of lead iodide. Pb2 (aq) 2I-(aq) PbI2(s) A student finds that 15.71 mL of 0.5770 M lead nitrate is needed to precipitate all of the iodide ion in a 25.00-mL sample of an unknown. What is the molarity of the iodide ion in the student's unknown

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1


Chemistry, 22.06.2019 00:30
Drive down any three characteristic of modern periodic table
Answers: 1

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1
You know the right answer?
The iodide ion concentration in a solution may be determined by the precipitation of lead iodide. Pb...
Questions

Mathematics, 11.05.2021 07:00

History, 11.05.2021 07:00

Mathematics, 11.05.2021 07:00

Biology, 11.05.2021 07:00

Computers and Technology, 11.05.2021 07:00

History, 11.05.2021 07:00





Mathematics, 11.05.2021 07:00

Mathematics, 11.05.2021 07:00

English, 11.05.2021 07:00

Mathematics, 11.05.2021 07:00

Biology, 11.05.2021 07:00

Mathematics, 11.05.2021 07:00

English, 11.05.2021 07:00

Mathematics, 11.05.2021 07:00

Mathematics, 11.05.2021 07:00