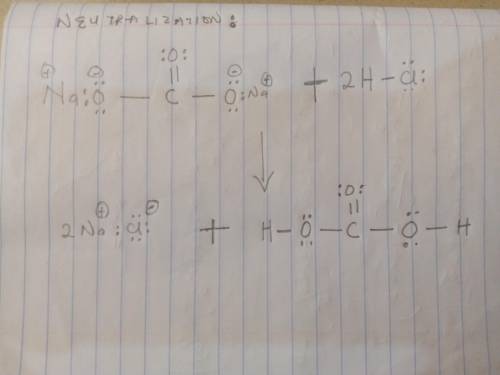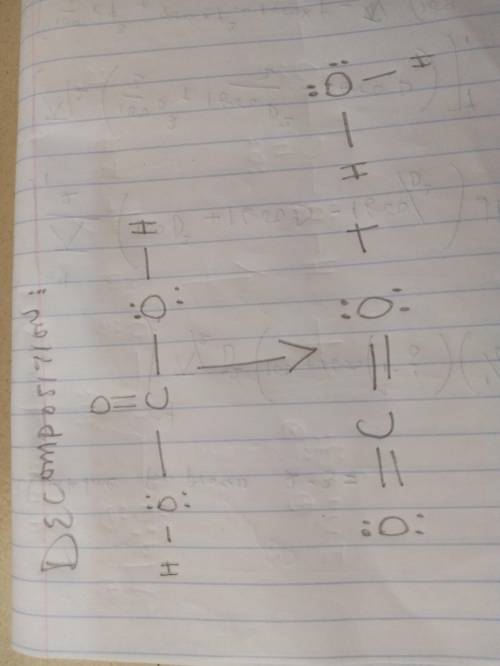Sodium bicarbonate is used to neutralize the remaining HCl at the end of the reaction. The initial products of this reaction are carbonic acid and sodium chloride. Carbonic acid then decomposes into carbon dioxide and water. Write a balanced chemical equation for each of these processes using Lewis structures (no molecular formulas).

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
What is a scientific theory? a. a scientist's guess about how something works b. the results of an experiment obtained using the scientific method c. a proven fact that will never change d. an idea that is backed by data from many sources
Answers: 2

Chemistry, 21.06.2019 18:10
Using complete sentences, explain how to predict the products and balance the reaction between sulfuric acid and potassium hydroxide.
Answers: 1

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1
You know the right answer?
Sodium bicarbonate is used to neutralize the remaining HCl at the end of the reaction. The initial p...
Questions



Mathematics, 11.04.2022 06:20


Biology, 11.04.2022 06:20


Mathematics, 11.04.2022 06:20

Mathematics, 11.04.2022 06:20


Geography, 11.04.2022 06:20

Business, 11.04.2022 06:20


History, 11.04.2022 06:30



Biology, 11.04.2022 06:30

Biology, 11.04.2022 06:30

Advanced Placement (AP), 11.04.2022 06:30