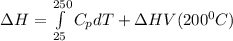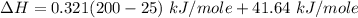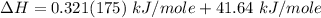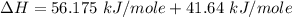Chemistry, 02.11.2020 16:30 monifaWilson
You are writing energy balances for a compound for which you cannot find heat capacity or latent heat data. All you know about the material are its molecular formula (C7H12N) and that it is a liquid at room temperature and has a normal boiling point of 200°C. Use this information to estimate the enthalpy of the vapor of this substance at 200°C relative to the liquid at 25°C.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2


Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 4.5 × 106 m/s. show your work. note: h= plank's constant (6.62607 x 10-34 j s)
Answers: 1

Chemistry, 23.06.2019 02:00
When an experimenter draws a conclusion that he assumes will apply to all situations set up similarly to his test situation, even though he cannot possibly have examined all possible test scenarios, the experimenter is using deductive reasoning inductive reasoning abductive reasoning subjective reasoning
Answers: 1
You know the right answer?
You are writing energy balances for a compound for which you cannot find heat capacity or latent hea...
Questions

Mathematics, 31.03.2021 04:30

Mathematics, 31.03.2021 04:30

Biology, 31.03.2021 04:30


Mathematics, 31.03.2021 04:30

Mathematics, 31.03.2021 04:30


Mathematics, 31.03.2021 04:30

Biology, 31.03.2021 04:30

Mathematics, 31.03.2021 04:30


Biology, 31.03.2021 04:30

Mathematics, 31.03.2021 04:30


Mathematics, 31.03.2021 04:30


Mathematics, 31.03.2021 04:30


Mathematics, 31.03.2021 04:30

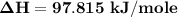
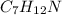
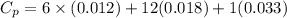
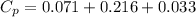
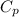 = 0.32 kJ/mole
= 0.32 kJ/mole