Chemistry, 02.11.2020 16:40 guzmangisselle
When I2(g) reacts with Cl2(g) to form ICl(g) , 26.8 kJ of energy are evolved for each mole of I2(g) that reacts.
Write a balanced thermochemical equation for the reaction with an energy term in kJ as part of the equation. Note that the answer box for the energy term is case sensitive.
Use the SMALLEST INTEGER coefficients possible and put the energy term (including the units) in the last box on the appropriate side of the equation. If a box is not needed, leave it blank.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

You know the right answer?
When I2(g) reacts with Cl2(g) to form ICl(g) , 26.8 kJ of energy are evolved for each mole of I2(g)...
Questions

Mathematics, 24.09.2019 03:50

Biology, 24.09.2019 03:50


History, 24.09.2019 03:50


Advanced Placement (AP), 24.09.2019 03:50


History, 24.09.2019 03:50



Mathematics, 24.09.2019 03:50

Social Studies, 24.09.2019 03:50

History, 24.09.2019 03:50

Social Studies, 24.09.2019 03:50

Health, 24.09.2019 03:50


Mathematics, 24.09.2019 03:50



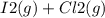 →
→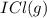
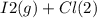 →
→ 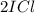
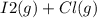 →
→