
Chemistry, 02.11.2020 21:00 SoccerdudeDylan
An aqueous magnesium chloride solution is made by dissolving 7.15 moles of MgCl2 in sufficient water so that the final volume of the solution is 2.50 L . Calculate the molarity of the MgCl2 solution.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1
You know the right answer?
An aqueous magnesium chloride solution is made by dissolving 7.15 moles of MgCl2 in sufficient water...
Questions

Social Studies, 11.03.2021 15:10



Social Studies, 11.03.2021 15:10

Mathematics, 11.03.2021 15:10


Mathematics, 11.03.2021 15:10


Mathematics, 11.03.2021 15:10



Business, 11.03.2021 15:10

Mathematics, 11.03.2021 15:10

English, 11.03.2021 15:10

Mathematics, 11.03.2021 15:10



Mathematics, 11.03.2021 15:10

Mathematics, 11.03.2021 15:10

Mathematics, 11.03.2021 15:10

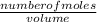
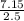 = 2.86mol/L
= 2.86mol/L

